electron configuration of zr|electron configuration calculator : Tuguegarao The total number of electrons in zirconium is forty. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons . Tingnan ang higit pa Living and Non-living drawing easy| How to draw Living and Non-living things Drawing#livingthings #nonlivingthings #easydrawing #livingthingsdrawing #nonlivi.
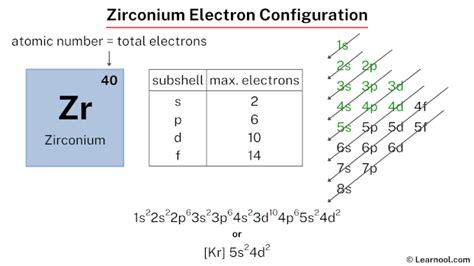
electron configuration of zr,The ground state electron configuration of zirconium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d2 5s2. This electron configuration shows that the last shell of zirconium has two electrons and the d-orbital has a total of two electrons. Therefore, the valence electrons of zirconiumare four. The elements . Tingnan ang higit paThe total number of electrons in zirconium is forty. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons . Tingnan ang higit paAtomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the nucleus is called the orbital. The sub-energy levels depend on the azimuthal quantum . Tingnan ang higit pa

Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the . Tingnan ang higit pa A step-by-step description of how to write the electron configuration for Zirconium (Zr). In order to write the Zr electron configuration we first need to know the number of .Electronic configuration of the Zirconium atom. Valence electrons. Orbital diagram.Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs. . Electron Configuration of Zirconium Zr Lesson. Moosing Chemistry. 1.46K subscribers. Subscribed. 9. 2K views 3 years ago Electron Configuration and Periodic . The standard form of Zirconium electron configuration is, however [Kr] 4d² 5s². How many valence electrons does Zirconium have? The Zr electron configuration is extremely useful in order to .
Electron configuration of Zirconium is [Kr] 4d2 5s2. Possible oxidation states are +4. Electron Configuration. The periodic table is a tabular display of the . The information on this page is fact-checked. Zirconium electron configuration | Image: Learnool. The zirconium electron configuration, denoted as [ Kr] 5s 2 4d 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 .Zirconium is a chemical element of the periodic table with chemical symbol Zr and atomic number 40 with an atomic weight of 91.2242 u and is classed as transition metal and is . Schematic electronic configuration of zirconium. The Kossel shell structure of zirconium. Atomic spectrum. A representation of the atomic spectrum of zirconium. Ionisation Energies and electron . Learn how to determine the electron configuration of zirconium Zr with this easy and clear lesson. Watch the video and follow the examples and exercises.
The electron configuration of Zr is highly useful so as to understand the element in a useful manner. With the Zirconium electron configuration, one can easily understand the electron distribution of . The zirconium electron configuration, denoted as 5s2 4d2 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d2, showcases the specific placement of electrons within the . Start from 1s and write till Zr for .The electronic configuration of Zirconium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d2 5s2. What is the abbreviated electronic configuration of Zirconium? The abbreviated electronic configuration of Zirconium is [Kr] 4d2 5s2. To form abbreviated notation of electronic configuration, the completely filled subshells are replaced by the noble gas . The electron configuration for the first row transition metals consists of 4s and 3d subshells with an argon (noble gas) core. . (Ni) and Zirconium (Zr) from the d-block. Nickel at Ground State: Ni: 8 d-electrons = [Ar] 4s 2 3d 8. Nickel with an Oxidation State of +2: Ni 2 +: [Ar] 4s 0 3d 8.Zirconium is a chemical element with symbol Zr and atomic number 40. Classified as a transition metal, Zirconium is a solid at room temperature. 40. Zr. Zirconium. Atomic Mass: 91.22 u: . Electron Configuration [Kr]5s 2 4d 2: Oxidation States +4: Year Discovered: 1789: View All Properties. 1 Identifiers.The same rule will apply to transition metals when forming ions. You should note that the ns electrons are always lost before the (n-1)d when forming cations for transition metals.For example, the electron configuration for Zn: [Ar]4s 2 3d 10 . the electron configuration for Zn +2: [Ar]3d 10 . The transition metals still do not end up being isoelectronic with a . The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number .electron configuration of zr electron configuration calculator Zr: properties of free atoms. Zirconium atoms have 40 electrons and the shell structure is 2.8.18.10.2. The ground state electron configuration of ground state gaseous neutral zirconium is [ Kr ]. 4d2. 5s2 and the term symbol is 3F2. Schematic electronic configuration of zirconium. The Kossel shell structure of zirconium.

Related Queries: average melting point, boiling point of metals vs nonmetals; electron configuration of zirconium; CAS number of zirconium; electronic configuration of group 4 elementselectron configuration of zrRelated Queries: average melting point, boiling point of metals vs nonmetals; electron configuration of zirconium; CAS number of zirconium; electronic configuration of group 4 elements An atom's electron configuration describes the way its electrons fill sublevels when the atom is in its ground state. Atoms seek the most stable electron configuration, so sublevels are half-filled or .Zirconium occurs in 6 natural isotopes: 90 Zr, 91 Zr, 92 Zr, 93 Zr, 94 Zr and 96 Zr. . Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. The .electron configuration calculatorElectron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and .Here [Ne] refers to the core electrons which are the same as for the element neon (Ne), the last noble gas before phosphorus in the periodic table. The valence electrons (here 3s 2 3p 3) are written explicitly for all atoms. Electron configurations of elements beyond hassium (element 108) have never been measured; predictions are used below. Consider the electronic structure of neutral iron and iron (III). To write the electronic structure for Fe 3 +: Fe: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2; Fe 3 +: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 5; The 4s electrons are lost first followed by one of the 3d electrons. This last bit about the formation of the ions is clearly unsatisfactory. The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter. For example; the electron configuration of zirconium is [Kr] 5s 2 4d 2. So the last electron of zirconium enters the d-subshell or d-orbital. Hence, zirconium is the d-block element.To elucidate the distribution of the electrons of an atom around its nucleus at various energy levels, its electron configuration is written. This configuration can be conveyed in the form {eq}\rm nl^e {/eq} where {eq}\rm n {/eq} shows the energy level or shell of the electron, {eq}\rm l {/eq} defines the subshell of an electron, and {eq}\rm e . To write electron configuration of an element, locate its symbol in ADOMAH Periodic Table and cross out all elements that have higher atomic numbers. For example, if you need to write electron configuration of Erbium (68), cross out elements 69 through 120. Notice numbers 1 through 8 at the base of the table.
electron configuration of zr|electron configuration calculator
PH0 · zr4+ electron configuration
PH1 · zr3+ electron configuration
PH2 · zr+2 electron configuration
PH3 · electron configuration guide
PH4 · electron configuration chart
PH5 · electron configuration calculator
PH6 · complete electron configuration for gold
PH7 · Iba pa